Iron is a well-known essential mineral that plays key roles in a variety of important processes in your body. You’ve probably been told by your parents or doctors about the dangers of iron deficiency, especially if you’re a woman. Perhaps the most important of the warnings is that without iron, your body wouldn’t make enough hemoglobin and myoglobin. And without these proteins? Your body wouldn’t get enough oxygen.
In a nutshell, iron is essential for life! Humans aren’t the only organisms that depend on iron either. Plants, animals, fungi, and bacteria rely on iron to transport oxygen and produce energy. Many bacteria and fungi even steal iron from their hosts.
So iron seems innocent enough. But can the phrase “too much of a good thing” apply to this highly desirable mineral? For people with hemochromatosis, the answer is “yes.” In fact, too much iron can be deadly. To find out why, we’ll take a deep dive into hemochromatosis.
What is Hemochromatosis?
Hemochromatosis, an iron overload disease, is one of the most common genetic diseases in the U.S., affecting as many as 1 out of every 200 Americans. There are four types of hemochromatosis, which are classified based on the age of onset and the genes involved:
- Type 1: Classic hemochromatosis, also known as hereditary hemochromatosis, HFE gene
- Type 2: Juvenile-onset hemochromatosis, HJV or HAMP gene
- Type 3: Transferrin receptor 2-related hereditary hemochromatosis, TFR2 gene
- Type 4: Ferroportin disease, SLC40A1 gene
Of the 4 types of hemochromatosis, types 1 through 3 are inherited in an autosomal recessive manner, and type 4 in an autosomal dominant pattern. An autosomal recessive inheritance pattern means that it typically takes two copies of the mutation in order for someone to be affected by the disorder. People with only one copy are called “carriers” of the gene and are thus unaffected. However, as we’ll see later, this may not always be the case.
For this article, we’ll focus on the most common type of hemochromatosis: Hereditary (type 1) hemochromatosis, which is mainly caused by mutations in the HFE gene on chromosome 6. Here’s an interesting side note: Unlike many other protein names, HFE is not an abbreviation of its official name, hemochromatosis. Instead, it stands of High Iron (chemical symbol: Fe). Creative, right?
The HFE gene encodes the HFE protein, which regulates the production of hepcidin. As the master regulator of iron homeostasis in the body, hepcidin acts by binding and degrading the transmembrane protein ferroportin to keep it from releasing too much iron into the bloodstream. However, when mutations in the HFE gene disrupt expression of hepcidin, too much iron can be released. Transferrin, which captures iron in plasma for transport into cells throughout the body, becomes highly saturated and excess iron is deposited into the liver.
Excess iron can have toxic effects due to the generation of reactive oxygen species (ROS). And since our bodies have no way to excrete iron, you can see why iron levels are so tightly regulated.
What Causes Hereditary Hemochromatosis?
Our bodies typically absorb between 5% to 35% of iron from our diet. However, when mutations are present in the HFE gene, there is no negative feedback mechanism to tell the body to stop absorbing iron. The main HFE mutations involved are:
- C282Y
- H63D
- S65C
As mentioned in the previous section, hereditary hemochromatosis is inherited in an autosomal recessive pattern. Knowing this, there are 3 ways that these mutations can be present:
- Homozygous: 2 copies of the mutation (Ex: C282Y/C282Y or H63D/H63D)
- Compound Heterozygous: 1 copy of C282Y and H63D each (C282Y/H63D)
- Heterozygous: 1 copy of either C282Y or H63D (Ex: C282Y/normal or H63D/normal)
Doctors often tell patients with hemochromatosis that there is no need to treat if they are not homozygous for the C282Y mutations. Indeed, the most severe cases of clinical iron overload are seen in C282Y homozygotes, and research studies have shown that C282Y/H63D compound heterozygotes have a lower risk of hemochromatosis-related morbidity. However, about 15% of the cases are compound heterozygotes or heterozygotes.
So if you’re carrier of the HFE mutations, you are still at risk of developing symptoms or complications from iron overload.
17 Symptoms of Hemochromatosis
Although hereditary hemochromatosis is present at birth, symptoms of hemochromatosis typically don’t occur until middle age – usually between 40 and 60 in men and after 50 in women. In its early stages, the symptoms of hemochromatosis are diverse and vague, so both patients and doctors mistakenly attribute them to other common illnesses, to menopause for women, or to “just getting old.”
To avoid a delayed or incorrect diagnosis, it’s important to be aware of the symptoms of hemochromatosis:
- Stomach pain, nausea
- Fatigue
- Joint pain
- Headache, dizziness
- Loss of sex drive
- Loss of body hair
- Lack of energy
- General weakness
- Weight loss
- Memory fog
- Irregular heart beat
- Restlessness
- Increased sensitivity to heat or cold
- Diabetes
- Impotence
- Insomnia
- Abnormal coloring of the skin (gray or bronze)
As a functional medicine practitioner, I teach patients that everything in our body is connected to one another. So it’s only natural that iron overload can also impact our emotional and mental well-being. Indeed, studies have shown that iron overload is associated with depression, irritability, and mood swings.
Menopause and Hemochromatosis – Is There A Link?
As I pointed out earlier, it appears that men are affected earlier and more commonly by hemochromatosis than women are. Many scientists and doctors believed (and still believe) that this occurs because women have an innate mechanism for shedding the excess iron – menstruation. Since men don’t lose blood every month, it only made sense that they would experience iron overload before women. And once women reach menopause and stop menstruating, that’s when they start experiencing symptoms of hemochromatosis.
This hypothesis was tested in a 1997 study that examined 176 women and 176 men with hemochromatosis. The researchers did find that women who stopped menstruating before the age of 50 (the average age of menopause) had higher iron buildup in their livers than women who continued having periods after 50.
However, they did not find that there was a difference in the age of symptom onset between men and women (48 and 50 respectively). And although men were shown to develop diabetes and cirrhosis more often, women were not immune to them as previously thought.
Here’s what I think was perhaps the most interesting finding of this study. The researchers concluded that although homozygous hemochromatosis is less common in women, menstruation and pregnancy did not appear to have any effect on the development of clinical symptoms. Therefore, doctors should suspect hemochromatosis in women with unexplained symptoms that are compatible with the disease to avoid delayed diagnosis and progressive tissue damage.
Effects of Hemochromatosis on Your Organs
From the list of symptoms above, it’s clear that hereditary hemochromatosis can affect multiple organ systems. In some cases, the following complications may be the first sign of hemochromatosis. However, not everyone will develop complications.
Here, we’ll delve a little bit deeper into the organs that are primarily affected by this disease.
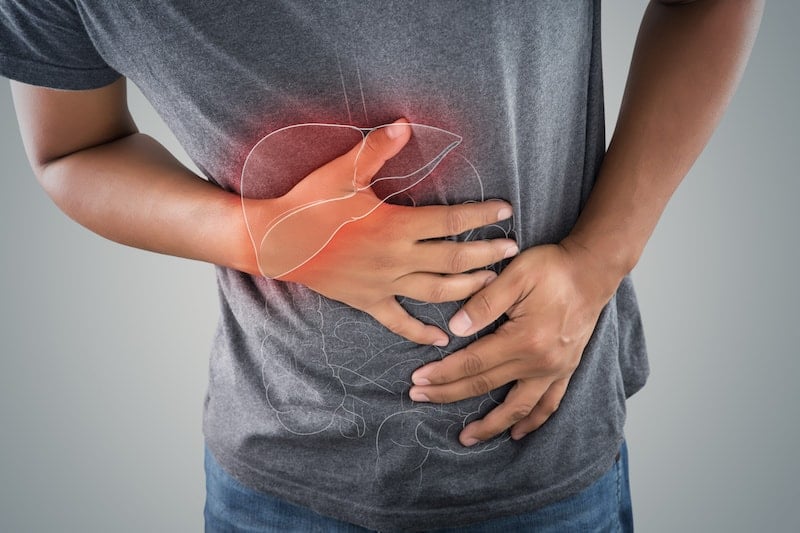
The Liver & Hemochromatosis
Your liver is a major storage organ of iron. And as the major site of iron storage, the liver can easily fall victim to iron overload. Enlarged liver, also known as hepatomegaly, is a common complication in early hemochromatosis. Hepatomegaly is followed by hepatic fibrosis, in which the liver shrinks due to scarring, cellular dysfunction and death, as well as other permanent damages. When such damage is widespread, it is diagnosed as cirrhosis. The scar tissue can block blood flow through your liver, preventing the organ from functioning normally. If cirrhosis is left untreated, it can lead to liver failure and liver cancer.
Unfortunately, most people are not aware that they have cirrhosis until their liver is badly damaged. If you have hereditary hemochromatosis, you should be aware of the following symptoms of cirrhosis:
- Tiredness or weakness
- Poor appetite
- Weight loss
- Digestive problems
- Pain in the upper right side of your abdomen
- Bruising and bleeding easily
- Confusion, mood changes, memory loss
- Sleep disorders
- Severe itchy skin
- Darkening of your urine
- Jaundice
Patients with cirrhosis due to hereditary hemochromatosis have a significantly higher risk of developing hepatocellular carcinoma. They also have a poorer survival rate following liver transplant, with only a 34% five-year post-transplant survival rate compared to the 70% seen in the general population.
Therefore, early diagnosis and treatment of hereditary hemochromatosis is essential for preventing the development of cirrhosis and reducing the risk of hepatocellular carcinoma.
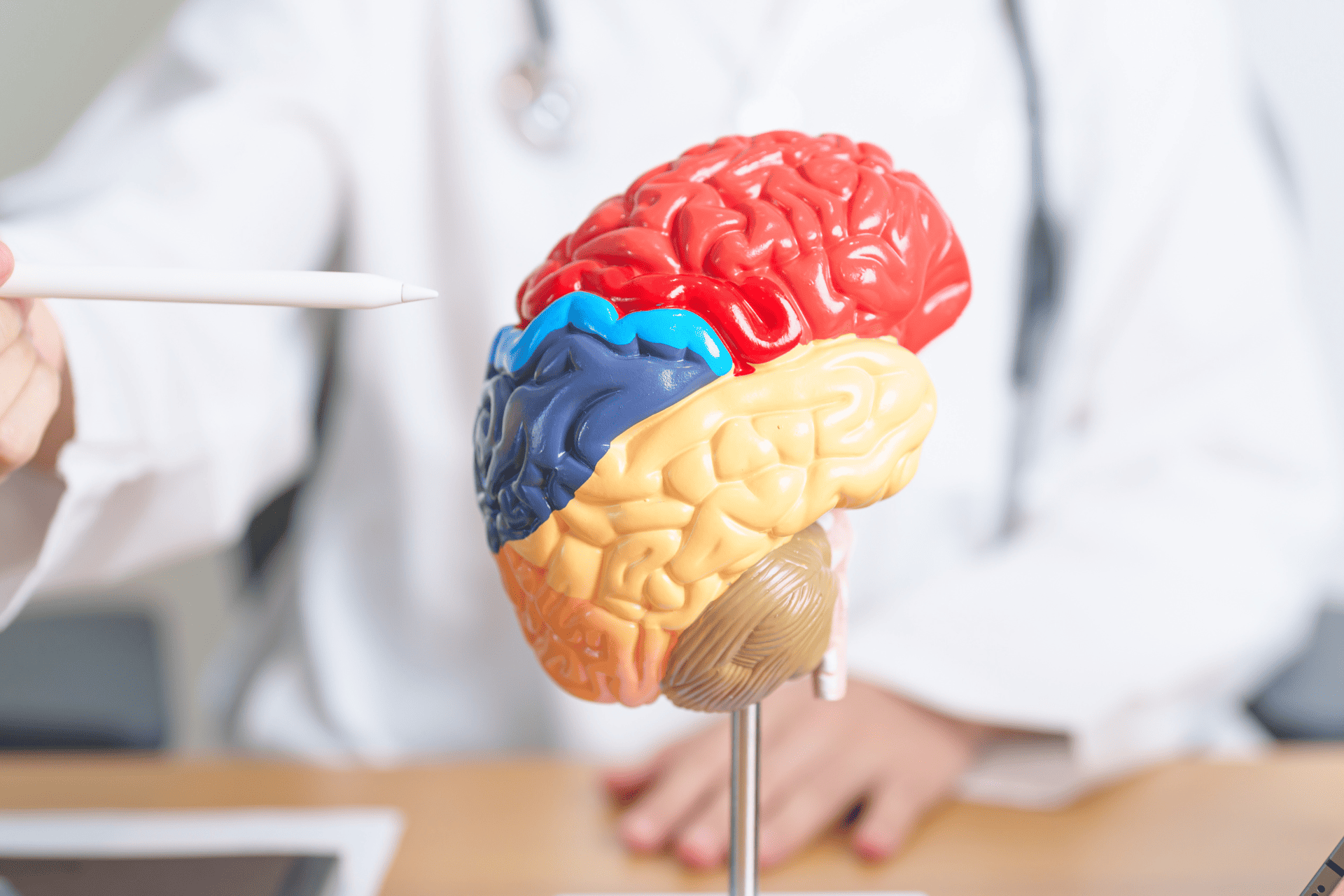
The Brain & Hemochromatosis
Iron is crucial for many biological processes of your brain, such as transport of oxygen, electron transfer, neurotransmitter synthesis, and myelin production. It’s such an important element that iron deficiency can cause impaired cognitive, language, and motor development in children. It is even associated with impaired dopamine metabolism in the brain.
So iron deficiency clearly has a negative effect on cognitive development. But what about iron overload?
While accumulation of iron in the brain is normal in the aging process, it turns out that iron overload due to hemochromatosis or impaired regulation can lead to chronic neurologic disorders, such as:
- Alzheimer’s disease
- Parkinson’s disease
- Multiple sclerosis
- Huntington’s disease
- Friedreich’s ataxia
- Amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease)
In patients with Alzheimer’s disease, for example, excessive iron has been shown to occur in neurons and two major pathological landmarks of the disease – neurofibrillary tangles and amyloid plaques. Furthermore, studies have shown that mutations in the transferrin and HFE genes, both of which lead to dysregulation of iron metabolism, appear more frequently in patients with Alzheimer’s disease.
How iron accumulates in the diseased brain is unknown. It’s also unclear whether the increased buildup of iron is a causative or contributing factor to the tissue damage in neurological diseases. But researchers have several suspicions for the ways iron is deposited into the brain, including:
- Hemorrhage/microbleeds
- Damaged cells
- Traumatic brain injury (TBI)
- Inflammatory processes
- Degradation of iron-related proteins (ceruloplasmin, ferroportin)
- Degradation of red blood cells and heme proteins
So can we somehow lower iron levels to prevent or slow down Alzheimer’s and other neurological diseases? Maybe. Small clinical studies have shown that iron chelators (drugs that bind to iron for removal) are capable of reducing levels of beta amyloid in the blood improving cognition in some patients.
The Heart & Hemochromatosis
Iron overload cardiomyopathy (IOC) is an increasingly common hemochromatosis complication. In the presence of iron overload, the left ventricle (LV) loses its ability to pump blood normally due to severe tissue damage. Untreated, LV dysfunction can lead to heart failure.
To understand how this happens, let’s look at the mechanism of iron buildup in the heart.
Under normal conditions, iron levels in the heart is regulated by the protein transferrin. Transferrin transports free iron in the blood to cells, where the iron eventually enters a pool of metabolically-active iron called labile free iron pool.
Excess iron in the labile free iron pool are bound to ferritin, the iron-storage protein. When cellular concentrations of iron rise, cells can accommodate by producing more ferritin. But they can only do this for so long. When the storage capacity of ferritin is exceeded, iron is released into the cells.
Initially, your heart is able to store the highly reactive free iron safely. However, when this capacity is exceeded, toxicity occurs through iron’s ability to generate free radicals, which damage various components of the cells, including proteins, lipids, and nucleic acids. These damages eventually lead to cell death and organ dysfunction.
The end result of this entire process is the development of a cardiomyopathy (heart muscle disease) characterized by moderate to severe LV dysfunction. Unfortunately, the prognosis is poor at this point, but patients have been known to improve with phlebotomy therapy.
The Pancreas & Hemochromatosis
Your pancreas has two main functions. As an exocrine gland, it secretes digestive enzymes to break down proteins, lipids, and carbohydrates. And as an endocrine gland, it secretes the hormones insulin and glucagon, which helps control your blood sugar levels.
Although iron accumulation in your pancreas tends to spare its exocrine functions, it can affect the endocrine functions, resulting in both type-2 and late-onset type-1 diabetes. Diabetes is more likely to develop in patients with a family history of diabetes. In one study, researchers reported that 25% of patients with both hemochromatosis and diabetes had first-degree relatives who also had diabetes.
In addition to family history of diabetes, the development of diabetes in patients with hemochromatosis likely involves multiple causative factors, including:
- Selective β-cell damage due to iron overload, which leads to decreased insulin secretion
- Insulin resistance through liver fibrosis
- Increased body mass index
- Metabolic syndrome (increased blood pressure, blood sugar, and body fat)
- Underlying genetic tendencies
The complications of diabetes in patients with hemochromatosis are the same as those that occur in patients without hemochromatosis and are unlikely to be resolved by hemochromatosis treatments like phlebotomy therapy. Therefore, management of diabetes in patients with hemochromatosis can follow the traditional recommendations.
The Pituitary & Hemochromatosis
While not as dangerous as liver or heart disease, iron accumulation can affect your pituitary glands, resulting in major hormone disturbances. Hypogonadism, or the failure of the gonads (testes in men, ovaries in women) to function properly, is perhaps the most disruptive in terms of quality of life for patients with hemochromatosis.
The hypogonadism in men in men can lead to:
- Body hair loss
- Testicular atrophy
- Muscle loss
- Low sex drive
- Erectile dysfunction
- Osteoporosis
- Secondary infertility
- Abnormal breast growth (gynecomastia)
- Anemia
- Diminished penile sensation
- Difficulty attaining orgasm
- Depression, irritability, and difficulty concentrating
- Hot flashes
- Abnormal cholesterol levels
Symptoms of hypogonadism in women include:
- Lack of menstruation
- Hot flashes
- Loss of body hair
- Low sex drive
- Secondary infertility
- Energy and mood changes
- Anemia
Fortunately, there is evidence that hypogonadism can be reversed with phlebotomy and hormone replacement therapy.
Bones, Joints, and Hemochromatosis
Arthropathy, a disease of joints, develops in between 25% and 50% of patients with hemochromatosis. Arthritis is a form of arthropathy that involves inflammation, but hemochromatosis arthropathy tends to be non-inflammatory and is a result of decades of iron deposition in joint cartilage.
First described in 1964, hemochromatosis arthropathy typically affects the hips and knees, although it can affect diarthrodial joints like the elbow, wrist, and thumb in some cases. This is the predominant cause of disability in patients with hemochromatosis and is the most commonly seen in C282Y homozygotes. However, C282Y heterozygotes are also affected albeit typically at a later age of onset.
Other musculoskeletal problems from iron overload include severe cramps and muscle pain.
Iron overload also plays a role in bone loss (osteopenia) and decreasing bone density (osteoporosis), particularly if the patient is also experiencing hypogonadism. In one study, researchers found that 25% and 41% of patients had osteoporosis and osteopenia, respectively, demonstrating that they are prevalent complications of the disease.
Although the mechanism of iron’s role in osteopenia and osteoporosis is unclear, laboratory experiments suggest that iron inhibits osteoblast function and disrupts the bone remodeling process. These activities eventually lead to a reduction in bone mineral density.
Hereditary Hemochromatosis Treatments
Although there is currently no cure for hemochromatosis, there are treatments that can provide significant relief of symptoms and complications by reducing the level of iron in your body.
Phlebotomy Therapy
Often referred to as the “gold standard” in hemochromatosis treatment, phlebotomy therapy is a procedure that removes blood from your body with the specific goal of reducing iron overload. Just as if you were donating blood, you visit a healthcare facility where a needle is inserted into a vein, and your blood is collected in a sterile container or bag.
The only differences between phlebotomy therapy and blood donation is that you need a doctor’s prescription for phlebotomy. In the beginning, your doctor may prescribe an intensive schedule of having your blood drawn once or twice a week, especially if your iron levels are very high (>1000 µg/L). For each pint (approx.. 470 mL) of blood, about 200 mg of iron is removed from your body, making phlebotomy a highly effective and safe hemochromatosis treatment. It may also reverse cirrhosis and improve left ventricular function in some cases.
While undergoing phlebotomy therapy, your serum ferritin (SF) levels should be monitored by your doctor. This is the most direct way to measure iron levels in your body. After the initial intensive period, if your SF levels are normal (≤200 µg/L in women, ≤300 µg/L in men), your doctor may decide to space out your treatments to once every 2 to 4 months.
Now, you may be wondering about what happens to all the blood taken out of you during phlebotomy treatment. Good news. Since hemochromatosis is not a transmissible condition, the FDA allows the blood from patients with hemochromatosis to be accepted for donation as long as:
- 1) The blood is labeled with the disorder
- 2) You receive an examination from a doctor at the time of donation if less than 8 weeks have passed since the previous donation
This is a win-win scenario! Donating blood saves not only your life, but someone else’s life, too. For a list of blood donation centers approved for collection of blood and blood products from patients with hemochromatosis, visit here.
Iron Chelation Therapy
For patients who are unable to tolerate phlebotomy therapy, doctors may recommend iron chelation therapy. Since iron typically is not excreted from our bodies after ingestion, this form of treatment involves the use of drugs called iron chelators, which can bind and remove excess iron from the body via urine or stool. Iron chelation therapy can either be injected or taken orally.
Early studies with iron chelators indicate that they are as effective as phlebotomy in removing iron. However, due to high cost, poor compliance, and adverse reactions, iron chelators may not be a satisfactory treatment for hemochromatosis.
6 Lifestyle and Dietary Recommendations for Hemochromatosis
In addition to blood removal, doctors may recommend dietary and lifestyle changes for patients with hemochromatosis. The following recommendations may slow down the rate of increase in iron stores and reduce the severity of hemochromatosis symptoms:
- Avoid iron supplements and multivitamins containing iron. They can increase your iron levels even more.
- Avoid raw fish and shellfish. Patients with hemochromatosis are more susceptible to infections caused by Vibrio vulnificus, a bacteria found in raw fish and shellfish from warm, coastal waters. Thoroughly cooked seafood is safe for consumption.
- Avoid contact of seawater with cuts or other open skin lesions. Same reason as above.
- Consume red meat in moderation. Red meat has levels of heme iron, which is more easily absorbed by your body than non-heme iron. Since patients with hemochromatosis already absorb more iron than normal, foods with high heme iron should be avoided.
- Limit vitamin C intake to 500 mg daily. Vitamin C increases iron absorption.
- Limit alcohol consumption. Drinking alcohol greatly increases the risk of liver disease in people with hemochromatosis. Patients with existing liver disease should avoid alcohol completely.
Living With Hereditary Hemochromatosis
If diagnosed and treated early, patients with hemochromatosis can live a normal life expectancy. If you have a family member with hemochromatosis or if you suspect that you are experiencing early symptoms of the disease, talk to your doctor about getting screened. Sometimes it’s not about getting old.
It’s true that iron is a vital element for our health. However, I see many patients with hereditary hemochromatosis at my practice, so I feel that it is important to be aware of its potential to do severe harm.
Are you surprised to learn about iron’s role as a double-edged sword? Do you have any experience with hereditary hemochromatosis? Share your thoughts in the comments below!
References:
http://www.diabetes.org/living-with-diabetes/complications/related-conditions/hemochromatosis.html
https://rarediseases.info.nih.gov/diseases/10746/hemochromatosis
https://ghr.nlm.nih.gov/condition/hereditary-hemochromatosis#definition
https://www.ncbi.nlm.nih.gov/pubmed/19554541
https://www.ncbi.nlm.nih.gov/pubmed/11479183
https://www.nhlbi.nih.gov/health-topics/hemochromatosis
https://www.mayoclinic.org/diseases-conditions/hemochromatosis/symptoms-causes/syc-20351443
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4555109/
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.850.6887&rep=rep1&type=pdf
https://www.niddk.nih.gov/health-information/liver-disease/cirrhosis/definition-facts
https://www.ncbi.nlm.nih.gov/pubmed/16083706/
https://www.ncbi.nlm.nih.gov/pubmed/15105265/
https://www.ncbi.nlm.nih.gov/pubmed/9507951/
https://www.ncbi.nlm.nih.gov/pubmed/12707938/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6306469/
https://www.ncbi.nlm.nih.gov/pubmed/14676042/
https://www.ncbi.nlm.nih.gov/pubmed/11430801/
https://link.springer.com/content/pdf/10.1007/BF00877096.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2947953/
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.111.050773
https://journals.sagepub.com/doi/pdf/10.1177/1358836X9900400207
https://www.ncbi.nlm.nih.gov/pubmed/11705485
https://www.sciencedirect.com/science/article/pii/0002934372900708
https://www.ncbi.nlm.nih.gov/pubmed/16538487/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5346371/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3255409/
https://medlineplus.gov/ency/article/001195.htm
https://www.ncbi.nlm.nih.gov/pubmed/11507369/
https://www.ncbi.nlm.nih.gov/pubmed/1392425/
https://www.sciencedirect.com/science/article/pii/S0002962915304948?via%3Dihub
http://www.jrheum.org/content/30/1/121.short
https://www.ncbi.nlm.nih.gov/pubmed/2919850?dopt=Abstract
https://link.springer.com/article/10.1007%2Fs00198-008-0701-4
https://www.ncbi.nlm.nih.gov/pubmed/23334864
https://www.ncbi.nlm.nih.gov/pubmed/6476075?dopt=Abstract
https://www.sciencedirect.com/science/article/pii/S0002962915304948?via%3Dihub
https://www.ncbi.nlm.nih.gov/pubmed/14632789
* These statements have not been evaluated by the Food and Drug Administration. The product mentioned in this article are not intended to diagnose, treat, cure, or prevent any disease. The information in this article is not intended to replace any recommendations or relationship with your physician. Please review references sited at end of article for scientific support of any claims made.
















14 Comments
Dr. Jill, thank you so much for this post! Hemochromatosis really is a silent killer. We have iron overload genes in both my parents families. I have inherited a couple of sets of the milder HFE genes. I am an advocate of genetic testing because I would never have known of my HFE genes, otherwise. I do have very elevated iron levels; and need to get my doctor to address this.
Hi Dr. Jill, I recently discovered an HFE heterozygous (H63D) mutation, via 23 and Me, and wonder if it could be related to some of my past and present symptoms. At age 35, I had premature ovarian failure, after many years of secondary infertility. I also had severe osteopenia/osteoporosis, leukopenia/neutropenia, severe fatigue, etc., at the same time. One year later, I did have a high iron level on a blood test. I currently have diastolic dysfunction, low digestive enzymes, mitochondrial dysfunction, etc., although recent bone marrow biopsy revealed low iron stores. I’m wondering if hemochromatosis could have contributed to my premature ovarian failure, 23 years ago.
Hi Lorraine
It is possible as iron can affect nearly any organ if it is stored in the wrong location
warmly
Dr Jill
WOW! Talk about timing!! My 19 yr old daughter that has previously been treated by you has high iron, high calcium and the doctors are stumped, as she does not have the gene for Hemochromotosis. She complained of increasing deep fatigue and debilitating brain fog, had two bone bruises to her pediatrician that brushed her off. Frustrated, I ran my own labs and discovered that her body has reason to feel so crappy. How can an Endocrinolgist & FP be so confounded by these albeit rare labs in a 19 yr old?
Thank you so much for this very informative article on heamochromotosis. I discovered l had it after my father died, in 2006. I was 57 then and my levels were very high. I have had lots of labotomies since and now within a safe range.
I am now 69 and have developed a lot of joint pains in my knees, wrists, ankles, finger joints, jaws and thumb base. I find it difficult to chew madium to hard foods.
I am under a rheumatologist at this moment also to help sort out what is going on.
Thanks again for this excellent post.
Should a high hemoglobin, like 15, in a woman trigger suspicion of hemochromatosis?
Have your doctor check iron levels
Hi Jill! Thanks so much for this information! Just wondering I am a female of 33 and I have often had higher iron levels during pregnancy and currently I’m not sure what my iron level is but what is typically a high iron level that would be a concern for someone my age? Thanks!!
Also thanks so much for your information on mold it saved my life!! So grateful!
It would be good to monitor and check for hemochromatosis gene
Hi Dr. Jill,
Thank you for posting this very comprehensive summary of Hereditary Hemochromatosis.
A stress thallium test revealed some narrowing in one of my coronary arteries. My cardiologist decided to run iron studies. Bingo…I had an elevated ferritin level. Following an MRI and genetic testing I I was diagnosed with HH (homozygous for C282Y gene) at 57. My brother was diagnosed a week later. We have regular phlebotomies.
My one comment: 3 years ago my liver specialist, (MD), sent me for an Abdominal Ultrasound with Elastography to determine the level of fibrosis in my liver due to HH. I had a repeat test last month with excellent results. This is a relatively new test which I noticed you did not mention in your blog.
I’m sorry I live in CT not CO. I’d make an appt to see you.
PS. Your segment on the Mold Summit was excellent.
All the best,
MF
67yr old female
Hi Dr. Jill,
I am a 48 yr old female-homozygous for H63D. I do not store iron in the typical fashion but I feel that the iron is causing so problems. I had 2 phlebotomies this year and started to avoid eating iron and now my ferritin has dropped to 16-I fear becoming anemic. With my iron panel looking good(except for low ferritin) I’m still symptomatic just be eating foods with iron. There are a few of us that are still having symptoms with good iron panels and our common factor is the MTHFR mutation. I also have histamine issues. I’m wondering if my symptoms are my body reacting to eating iron and then having histamine issues and if the MTHFR mutation is playing some part in all of this. Would love to have a phone consult with you.
Dr. Jill,
Thank you for such an informative article. I am recently diagnosed and putting so many pieces of my health puzzle together. I began to have extreme fatigue after my second son was born, (my cortisol, dhea, pregnelone and sex hormones tanked), pain and fatigue after exercise verses energy and feeling strong, hypothyroid, low insulin and chromium, low on so many minerals/vitamins, I have had damaged optic nerves for many years and now vision loss (but my eye pressure was normal). I had a hysterectomy and now 2 years later diagnosed with iron overload. I am wondering if hemochromotosis is the main puzzle piece. I am only 47, and have been diagnosed with all the above in just the latest 10 years. I have some fibrosis in my liver as well. Prior to having kids at 29 and 35, I never went to the doctor other than for annual physicals. Thank you again for your article! I have saved it to my home screen to share with family in hopes my siblings will get tested.
I appreciate your article greatly. After being diagnosed a year ago, I have only found the same, vague information about it. Your article goes into the depths of the disease and all major organs. The sub categories and their symptoms comparing men and women is especially helpful. Thank you so much for this!
Dr. Jill-
Thank you so much for writing this informative (very clear) article. I have recently been diagnosed with compound heterozygous hemochromatosis. I have been suffering many symptoms for years. My ferritin is not high(109) but my iron sat is 66%, iron is 170 and UIBC is low at 87. I have not seen my hematologist yet but have been told by someone that my numbers do not represent hemochromatosis!? Can you weigh in on this? Again, thank you for the really great article here.
Tricia Yanek
Share: